Your Coordination number of face centered cubic images are ready in this website. Coordination number of face centered cubic are a topic that is being searched for and liked by netizens now. You can Get the Coordination number of face centered cubic files here. Find and Download all free images.
If you’re searching for coordination number of face centered cubic pictures information linked to the coordination number of face centered cubic interest, you have visit the right site. Our site frequently provides you with suggestions for downloading the highest quality video and image content, please kindly surf and find more enlightening video articles and graphics that fit your interests.
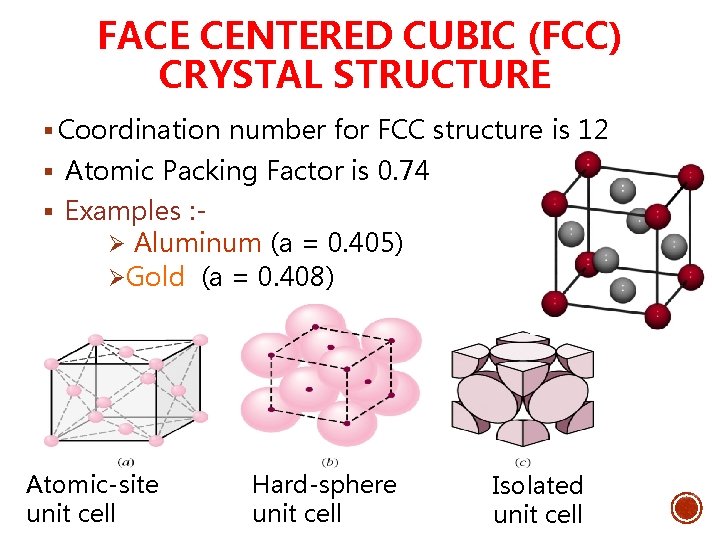
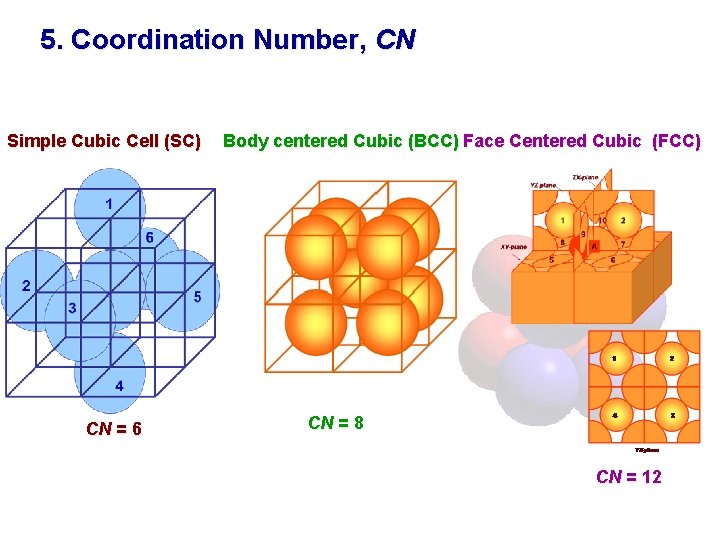
Coordination Number Of Face Centered Cubic. Coordination Number of Simple Cubic Crystal Structure is 6 Coordination Number of Body Centered Crystal Structure BCC is 8 Coordination Number of Face Centered Crystal Structure FCC is 12 Coordination Number of Hexagonal close packed structure HCP is 12 Know more about the basic types of crystal structures. Face Centered Cubic FCC Structure Each of the corner atoms is the corner of another cube so the corner atoms are shared among eight unit cells. In a body-centered cubic crystal each atom has 8 nearest neighbors NN. Each face-centered atom is shared by two surround unit cells.
 Week 5 Crystal Structure In Material Sem 2 From slidetodoc.com
Week 5 Crystal Structure In Material Sem 2 From slidetodoc.com
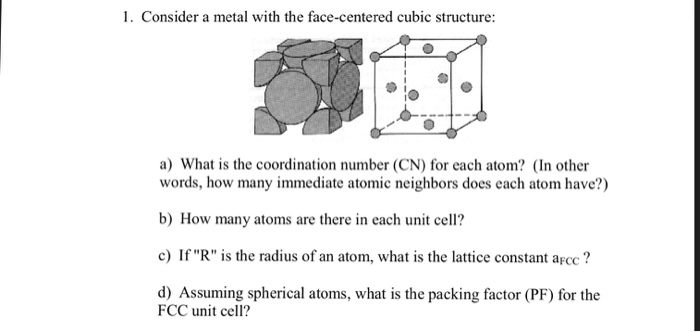
The body - centered cubic bcc has a coordination number of 8 and contains 2 atoms per unit cell. In addition there are 6 atoms at the face centers of the cube. Since the center atom is in contact with eight other atoms we say that it has a coordination number of eight. For any corner atom of the unit cell the nearest atoms are face-centred atoms. The coordination number for a face-centered cubic structure is the number of nearest-neighbor atoms or ions surrounding an atom or ion in that structure. Face-centered cubic FCC or cF is the name given to a type of atom arrangement found in nature.
Coordination Number of Simple Cubic Crystal Structure is 6 Coordination Number of Body Centered Crystal Structure BCC is 8 Coordination Number of Face Centered Crystal Structure FCC is 12 Coordination Number of Hexagonal close packed structure HCP is 12 Know more about the basic types of crystal structures.
For any corner atom of the unit cell the nearest atoms are face-centred atoms. Face-centered cubic FCC or cF is the name given to a type of atom arrangement found in nature. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works. Since 12 of its atoms are shared it is said to have a coordination number of 12. The coordination number of a face centered cubic space lattice is. Complete step by step answer.
 Source: youtube.com
Source: youtube.com
Hence in order to solve these types of questions one can understand the face-centered cubic structure of copper and find out the coordination number. The body - centered cubic bcc has a coordination number of 8 and contains 2 atoms per unit cell. Face-centered cubic FCC or cF is the name given to a type of atom arrangement found in nature. The face- centered cubic FCC has a coordination number of 12 and contains 4 atoms per unit cell. Since the face atoms are shared by two cubes they only contribute three net atoms to.
What is the coordination number of a face centered cubic FCC unit cell. For any corner atom of the unit cell the nearest atoms are face-centred atoms. Additionally each of its six face centered atoms is shared with an adjacent atom. Hence the number of face centered atoms in unit cell 12 x 6 3 atoms. Face Centered Cubic FCC Structure Each of the corner atoms is the corner of another cube so the corner atoms are shared among eight unit cells.

Coordination Number of Simple Cubic Crystal Structure is 6 Coordination Number of Body Centered Crystal Structure BCC is 8 Coordination Number of Face Centered Crystal Structure FCC is 12 Coordination Number of Hexagonal close packed structure HCP is 12 Know more about the basic types of crystal structures. Complete step by step answer. The face- centered cubic FCC has a coordination number of 12 and contains 4 atoms per unit cell. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works. Hence the number of face centered atoms in unit cell 12 x 6 3 atoms.

Face-Centered Cubic FCC Structure Unit cell contains. Face Centered Cubic This unit cell uses 14 atoms eight of which are corner atoms forming the cube with the other six in the center of each of the faces. 5 rows The face-centered cubic fcc has a coordination number of 12 and contains 4 atoms per. 6 x 12 8 x 18 4 atomsunit cell Coordination number 12 Close packed directions are face diagonals. A face-centered cubic unit cell structure consists of atoms arranged in a cube where each corner of the cube has a fraction of an atom with six additional full atoms positioned at the center of each cube face.
 Source: dornshuld.chemistry.msstate.edu
Source: dornshuld.chemistry.msstate.edu
What is the coordination number of a face centered cubic FCC unit cell. The simple cubic has a coordination number of 6 and contains 1 atoms per unit cell. A face-centered cubic unit cell structure consists of atoms arranged in a cube where each corner of the cube has a fraction of an atom with six additional full atoms positioned at the center of each cube face. Face-centered cubic FCC or cF is the name given to a type of atom arrangement found in nature. Coordination Number of Simple Cubic Crystal Structure is 6 Coordination Number of Body Centered Crystal Structure BCC is 8 Coordination Number of Face Centered Crystal Structure FCC is 12 Coordination Number of Hexagonal close packed structure HCP is 12 Know more about the basic types of crystal structures.
 Source: slidetodoc.com
Source: slidetodoc.com
Coordination number of Face Centered Cubic - YouTube. The face- centered cubic FCC has a coordination number of 12 and contains 4 atoms per unit cell. That is not the maximum which is 12 found in close-packed structures but BCC has such high stability because of its next-nearest neighbors. Each face-centered atom is shared by two surround unit cells. Complete step by step answer.

In addition there are 6 atoms at the face centers of the cube. In face centred cubic lattice any atom present in FCC lattice touches 12 other atoms hence the coordination number in FCC unit cell is 12. Thus the coordination number for an FCC structure 4. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works. Coordination Number CN is the number of nearest neighbors that each atom has.
 Source: youtube.com
Source: youtube.com
The atoms at the corner of the cube are shared with eight. A 4 b 6 c 8 d 12 Answer. Click here to view the lattice. Coordination Number of Simple Cubic Crystal Structure is 6 Coordination Number of Body Centered Crystal Structure BCC is 8 Coordination Number of Face Centered Crystal Structure FCC is 12 Coordination Number of Hexagonal close packed structure HCP is 12 Know more about the basic types of crystal structures. -First of all lets understand the.
 Source: sciencedirect.com
Source: sciencedirect.com
In addition there are 6 atoms at the face centers of the cube. In a unit cell an atoms coordination number is the number of atoms it is touching. Click here to view the lattice. In a body-centered cubic crystal each atom has 8 nearest neighbors NN. In an FCC structure there are eight atoms one atom each at the corner of the unit cell and one atom at the centre of each face.
 Source: examhill.com
Source: examhill.com
This video explains coordination number of BCC in a brief manner. In addition there are 6 atoms at the face centers of the cube. -First of all lets understand the. This video explains coordination number of BCC in a brief manner. In a body-centered cubic crystal each atom has 8 nearest neighbors NN.
 Source: slidetodoc.com
Source: slidetodoc.com
That is not the maximum which is 12 found in close-packed structures but BCC has such high stability because of its next-nearest neighbors. Face-centered cubic FCC or cF is the name given to a type of atom arrangement found in nature. Thus the coordination number for an FCC structure 4. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works. The hexagonal closest packed hcp has a coordination number of 12 and contains 6 atoms per unit cell.

Since 12 of its atoms are shared it is said to have a coordination number of 12. 5 rows The face-centered cubic fcc has a coordination number of 12 and contains 4 atoms per. Face Centered Cubic This unit cell uses 14 atoms eight of which are corner atoms forming the cube with the other six in the center of each of the faces. Hence in order to solve these types of questions one can understand the face-centered cubic structure of copper and find out the coordination number. Face Centered Cubic FCC Structure Each of the corner atoms is the corner of another cube so the corner atoms are shared among eight unit cells.

That is not the maximum which is 12 found in close-packed structures but BCC has such high stability because of its next-nearest neighbors. Additionally each of its six face centered atoms is shared with an adjacent atom. Face-Centered Cubic FCC Structure Unit cell contains. The body - centered cubic bcc has a coordination number of 8 and contains 2 atoms per unit cell. In face centred cubic lattice any atom present in FCC lattice touches 12 other atoms hence the coordination number in FCC unit cell is 12.
 Source: youtube.com
Source: youtube.com
Since 12 of its atoms are shared it is said to have a coordination number of 12. The hexagonal closest packed hcp has a coordination number of 12 and contains 6 atoms per unit cell. Additionally each of its six face centered atoms is shared with an adjacent atom. That is not the maximum which is 12 found in close-packed structures but BCC has such high stability because of its next-nearest neighbors. Each face-centered atom is shared by two surround unit cells.

The body - centered cubic bcc has a coordination number of 8 and contains 2 atoms per unit cell. The face-centered cubic fcc has a coordination number of 12 and contains 4. Hence the number of face centered atoms in unit cell 12 x 6 3 atoms. In a unit cell an atoms coordination number is the number of atoms it is touching. In an FCC structure there are eight atoms one atom each at the corner of the unit cell and one atom at the centre of each face.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title coordination number of face centered cubic by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






